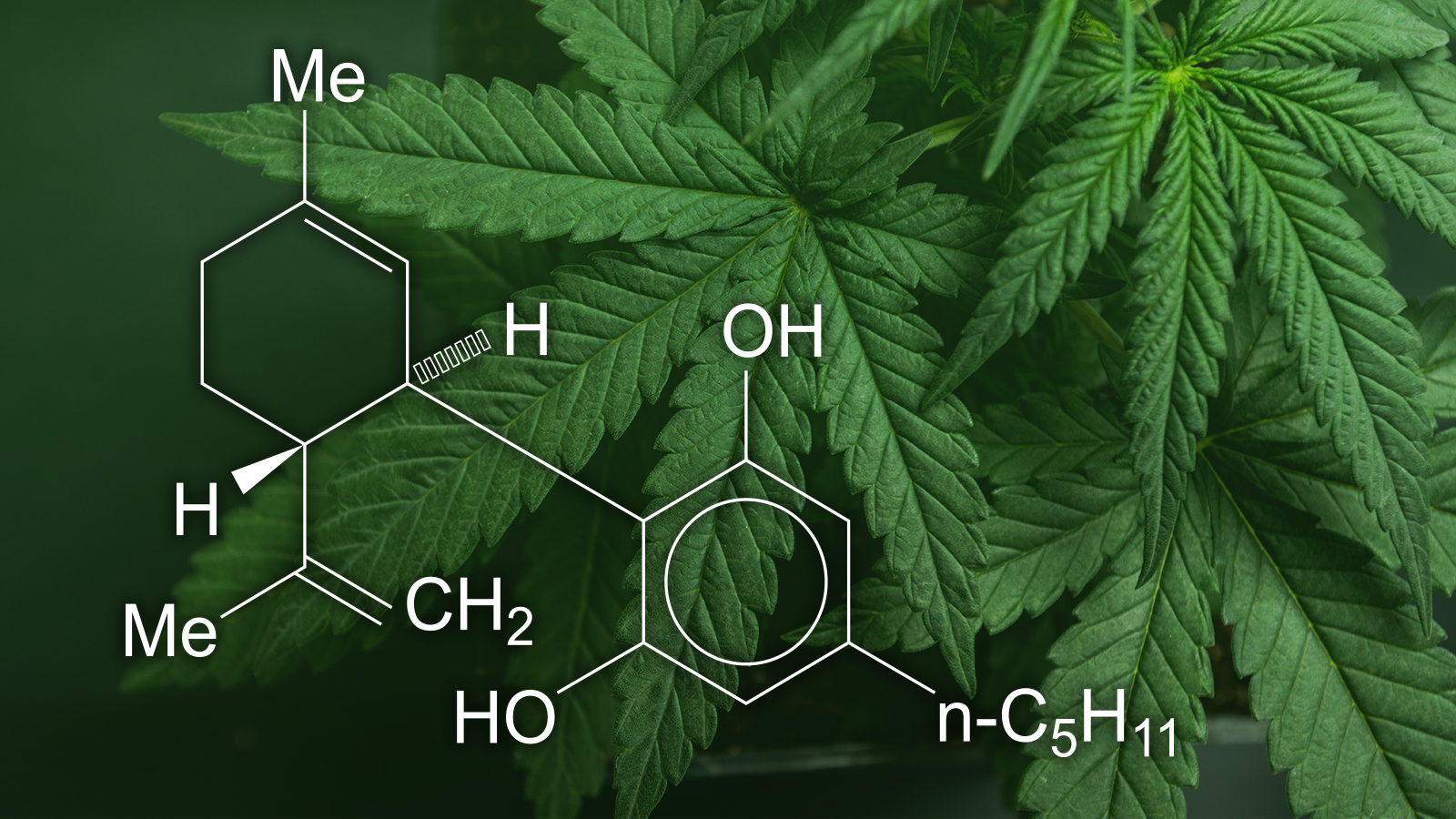
CBD has been attributed to successfully treating a series of problems from epileptic seizures and insomnia to pain as well as inflammation. Yet, there’s little clinical proof for the majority of these advantages.
Now, one use for CBD has adequate evidence to call for authorization for clinical treatment. In June 2018, the FDA accepted a CBD-based medication called Epidiolex as a therapy for numerous serious forms of uncommon childhood years epilepsy. It’s the initial government-approved for clinical use for CBD.
To learn about the variety of hemp flower strains, please follow the link.
That does not indicate CBD isn’t high up on the research radar. The Nat trials database provides greater than 150 tests involving CBD that are presently active or in the procedure of recruiting.
Scientists located that cannabidiol eliminated all stress of effective bacteria they checked, consisting of some responsible for several serious infections that have been most resistant to existing prescription antibiotics. They think cannabidiol “is a promising new antibiotic worth additional investigation. The findings were displayed in June of 2019 at the yearly conference of the American Society for Microbiology, as well as haven’t been published in peer-reviewed research.
CBD is one of the most appealing drugs that has appeared for neuropsychiatric illness in the last half a century. It is claimed by a scientist who is collaborating on a research study of CBD as a therapy for post-traumatic stress disorder as well as alcohol implanted disorders in the body. The reason it is so encouraging is that it has a one-of-a-kind combination of safety as well as effectiveness across of extremely broad series of problems.
On Might 31, the FDA held its first public hearing about the safety of products containing cannabis. The objective of the hearing was to “determine and collect all offered data to aid us to answer these concerns in order to ensure that the American public is shielded, including to the level of CBD is being presented into our food supply or other usual customer items.
Market watchers are confident that the FDA will develop policies for items to ensure that manufacturers cannot make false claims or disperse hazardous things.
There needs to be clear action in notifying the public that there is no science behind the generic claims made.
If you want to find more about CBD, you should go through the varieties of researches carried on CBD. To know about the advantages and uses of CBD, please visit the page https://theholbornmag.com/what-are-the-advantages-as-well-as-uses-of-cbd-oil/.










Comments